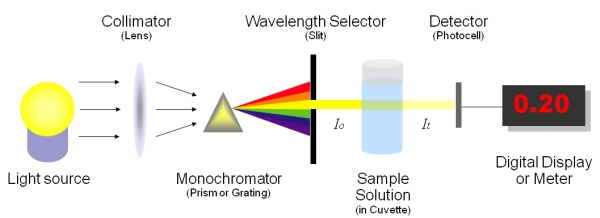
Spectrophotometry
Figure 1: principles of spectrophotometry (Source: Gore, Michael. Spectrophotometry & Spectrofluorimetry. New York: Oxford University Press, 2000)
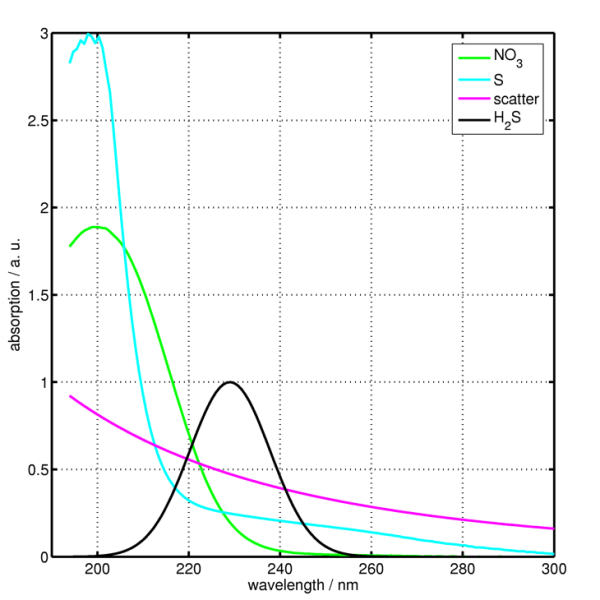
Previous studies relating to the determination of nitrate by UV absorption showed promising methods for continuous monitoring. The work from Aztec Environmental Control Ltd. published an article titled “On-line Nitrate Water Quality Monitor for Department of the Environment” (1988). Their work showed, that if the UV absorption band was measured not at 210 nm (the peak for nitrate), but at a longer wavelength (up to 225 nm) then interferences from other inorganic anions was reduced and correction for the effect of organic matter became more reliable. The correction at 225 nm needed to compensate for UV absorption by organic matter was found by extrapolation back from its absorption at wavelengths above 250 nm where nitrate does not absorb 1.
Application: Cadmium Reduction Method

The cadmium reduction method is a colorimetric method that involves contact of the nitrate in the sample with cadmium particles, which cause nitrates to be converted to nitrites. The nitrites then react with another reagent to form a red color whose intensity is proportional to the original amount of nitrate. The red color is then measured either by comparison to a color wheel with a scale in mg/L that increases with the increase in color hue, or by use of an electronic spectrophotometer that measures the amount of light absorbed by the treated sample at a wavelength of 543 nm. The absorbance value is then converted to the equivalent concentration of nitrate by using a standard curve. This method requires that samples being treated are clear. If a sample is turbid, it should be filtered and made sure that the filter is nitrate-free. If concentrations of copper, iron, or other metals are present, the reaction with cadmium will be slowed down and the reaction time will have to be increased 2.
The reagents used for this method are often prepackaged for different ranges, depending on the expected concentration of nitrate in the stream. For example, the Hach Company provides reagents for the following ranges: low (0 to 0.40 mg/L), medium (0 to 4.5 mg/L), and high (0 to 30 mg/L).